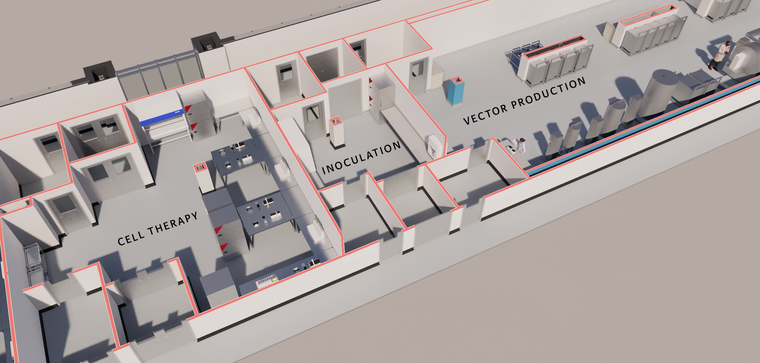
FACILITIES FOR CELL & GENE THERAPY
One of the hottest topics in the pharmaceutical industry today is cell and gene therapy, or, as it is referred to in Europe, ATMP (Advanced Therapy Medicinal Products). Often included in these discussions are industry concerns about the shortage in capacity for virus vector manufacturing. Our familiarity designing these facilities over the last six years indicates that, from a facility point of view, instead of the broad term cell and gene therapy, it is more instructive to talk about facilities for allogeneic or autologous therapies. These are better descriptors because they communicate important aspects about the size and nature of the manufacturing process and, consequently, the manufacturing facility that is needed to support these exciting products.
Read More