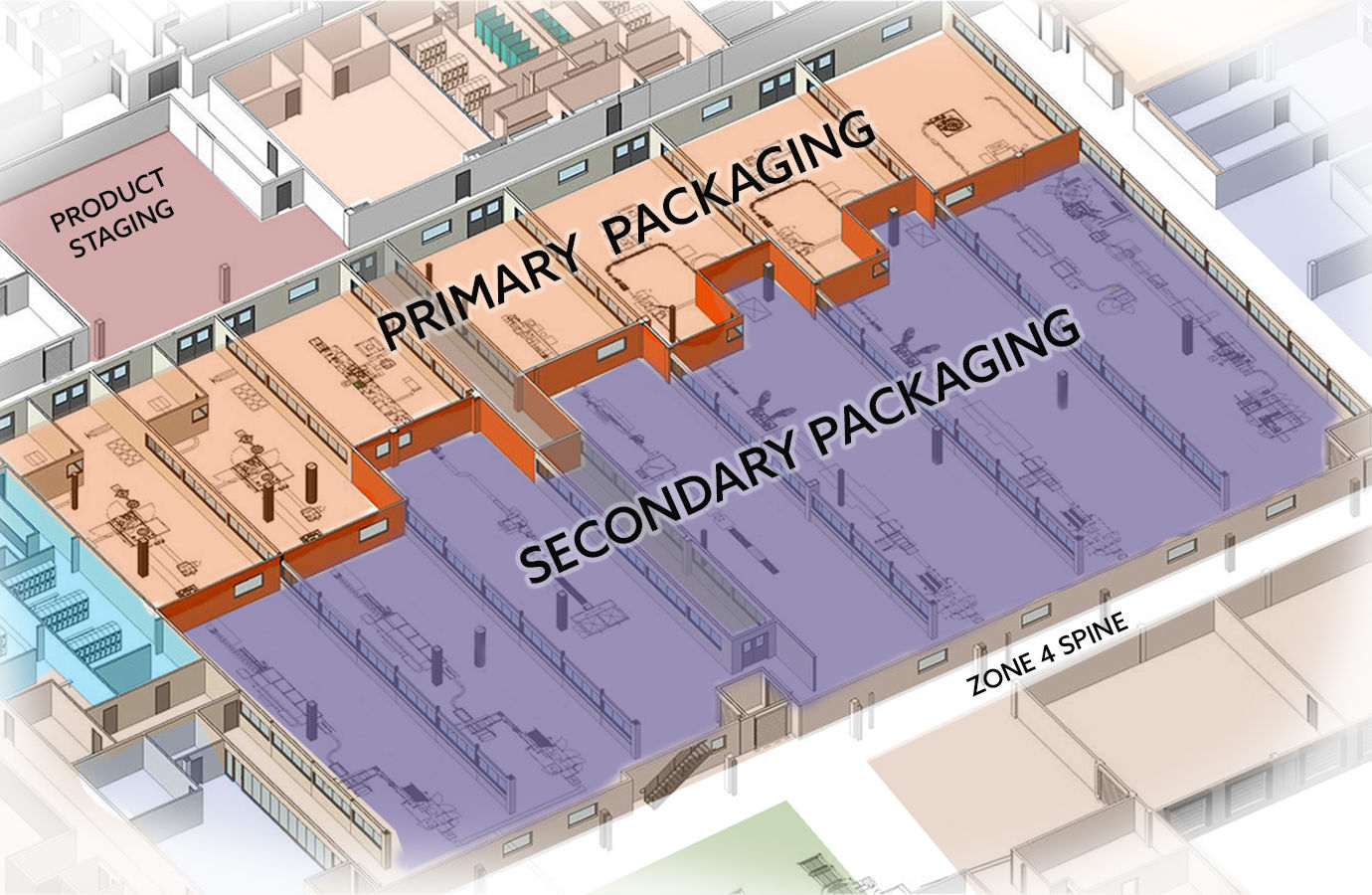
The condition of this existing over-the-counter manufacturing facility, first built in 1963, was the result of many years of additions and modifications. As a consequence, the plant was not fully compliant with modern and internal corporate GMPs. Indicators included staging and production zones being dispersed and isolated, and inconsistent gowning and hygiene zoning standards.
JacobsWyper was approached to provide a master plan to transform this legacy facility into a modern plant with compliant flows, segregation and cGMP practices. This was achieved by developing a plan that reallocated assets to transform the quality standards, employee culture and product portfolio to focus solely on the manufacture and packaging of solids and powders. The program included renovation of 295,000 sf of manufacturing and packaging, as well as 12,000 sf of lab renovations that included a 2,000 sf microbiology laboratory. The new plan achieves an efficient layout that is compliant with the Owner’s standards, the FDA, EU and other global regulatory agencies.